Transition Metal Signaling and Metalloallostery
Bioinorganic Chemistry Beyond Active Sites
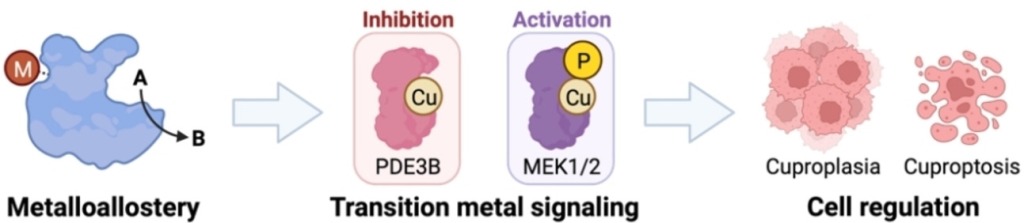
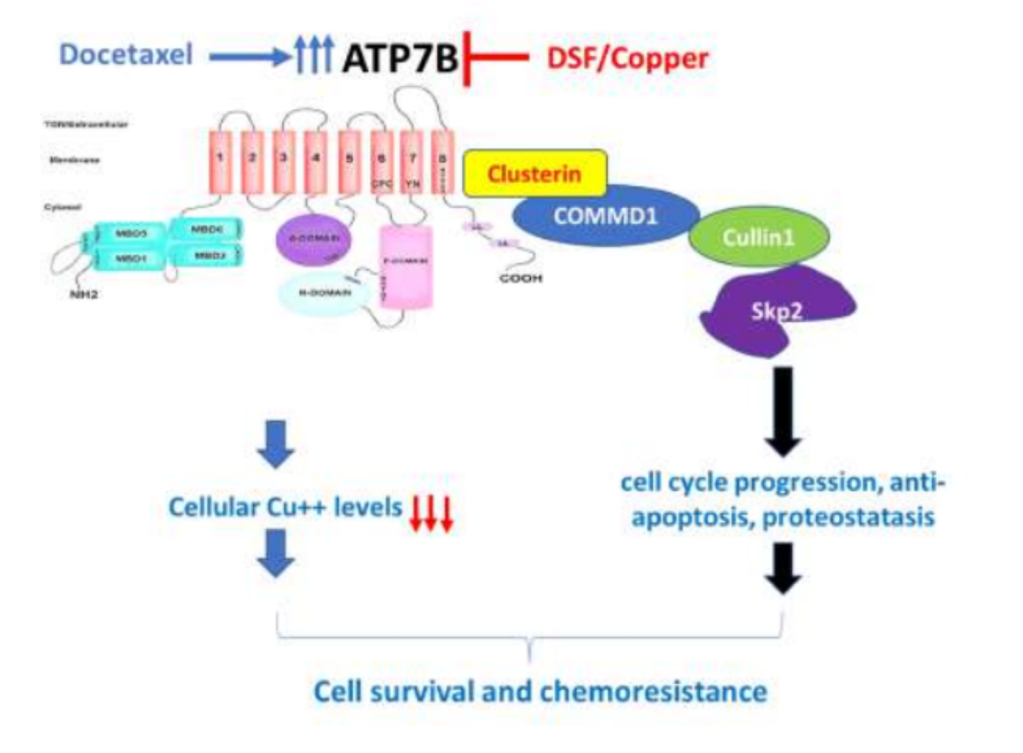
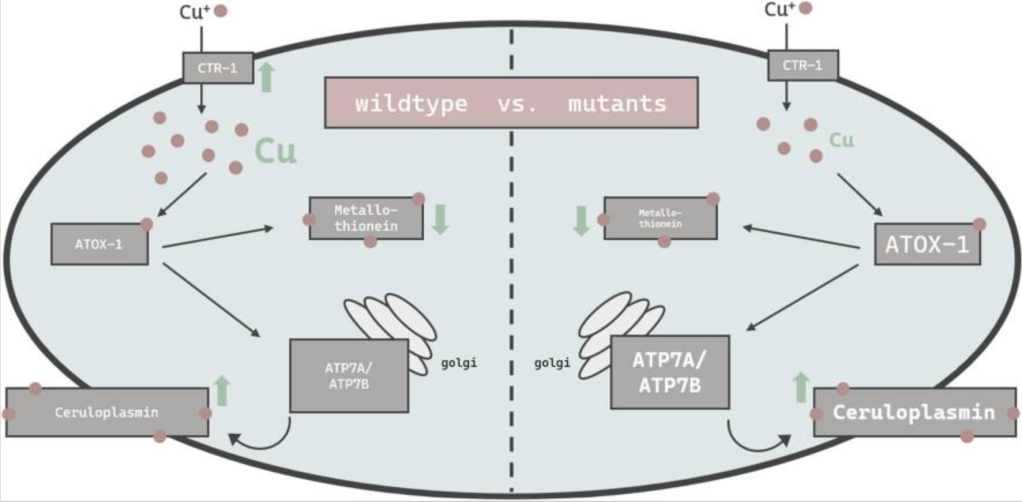
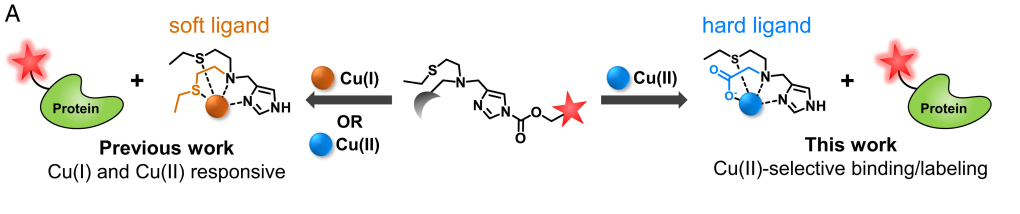
We are advancing a new paradigm of transition metal signaling, where metal nutrients like copper and iron can serve as dynamic signals to regulate protein function by metalloallostery, going beyond their traditional roles as static active site cofactors. We develop activity-based sensing probes for imaging mobile transition metal pools and activity-based proteomics probes for identifying allosteric metal sites in proteins. These chemical tools enable us to decipher the complex biology of sleep, cognition, and obesity in cell, zebrafish, and mouse models. We also develop medicines to target metals as disease vulnerabilities in cancer, neurodegeneration, and metabolic liver disorders. These drug discovery efforts focus on cuproplasia and cuproptosis, newly recognized forms of copper-dependent cell proliferation and cell death, respectively.